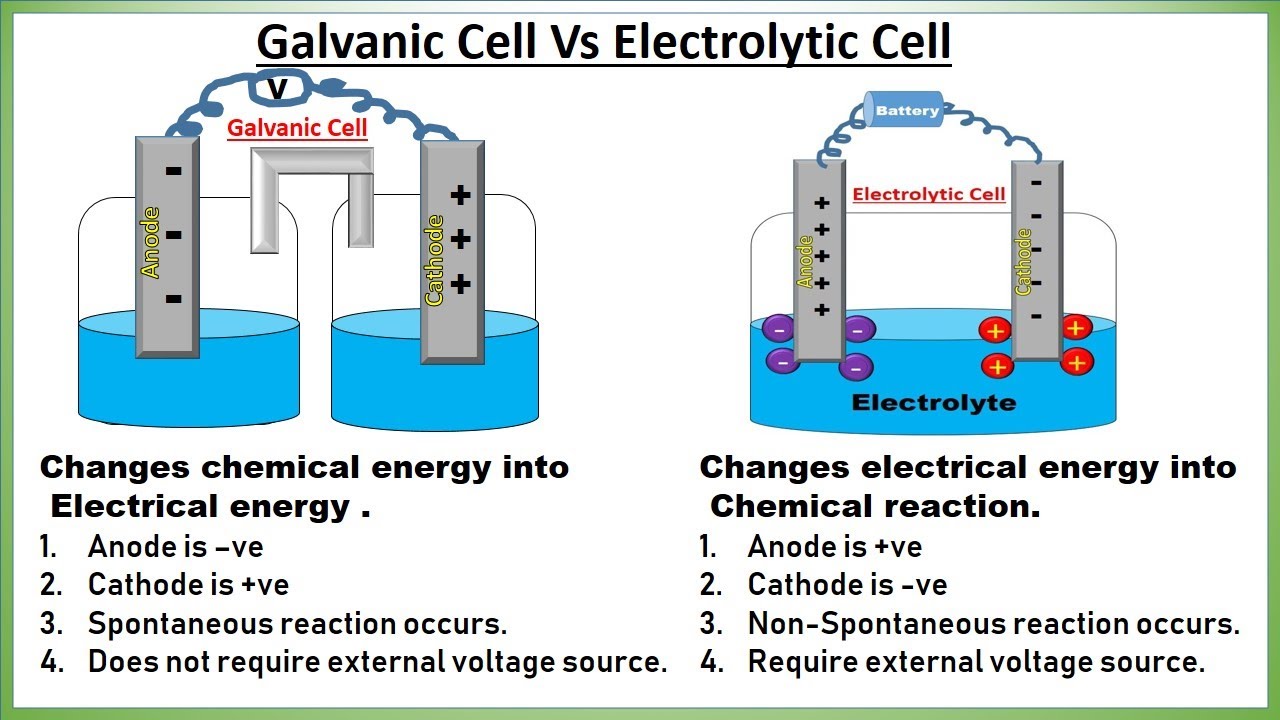
Similarities Between Galvanic And Electrolytic Cells . The table shows additional similarities and. Both cells have electrical current flowing. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. — the galvanic cell and electrolytic cells have some similarities. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic.
from www.youtube.com
The table shows additional similarities and. — the galvanic cell and electrolytic cells have some similarities. — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. Both cells have electrical current flowing. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the.
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells
Similarities Between Galvanic And Electrolytic Cells a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. — the galvanic cell and electrolytic cells have some similarities. The table shows additional similarities and. — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. Both cells have electrical current flowing.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How Similarities Between Galvanic And Electrolytic Cells The table shows additional similarities and. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. — the galvanic cell and electrolytic. Similarities Between Galvanic And Electrolytic Cells.
From schoolbag.info
Electrochemistry Review the Knowledge You Need to Score High 5 Similarities Between Galvanic And Electrolytic Cells — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. — the galvanic cell and electrolytic cells have some similarities. The table shows additional similarities and. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. electrons in galvanic cells (several. Similarities Between Galvanic And Electrolytic Cells.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell Similarities Between Galvanic And Electrolytic Cells The table shows additional similarities and. Both cells have electrical current flowing. — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. — electrolytic cells are very similar to voltaic (galvanic) cells. Similarities Between Galvanic And Electrolytic Cells.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Similarities Between Galvanic And Electrolytic Cells — the galvanic cell and electrolytic cells have some similarities. The table shows additional similarities and. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. Both cells have electrical current flowing. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous. Similarities Between Galvanic And Electrolytic Cells.
From www.differencebetween.net
Difference Between Galvanic Cells and Electrolytic Cells Difference Similarities Between Galvanic And Electrolytic Cells a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt. Similarities Between Galvanic And Electrolytic Cells.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog Similarities Between Galvanic And Electrolytic Cells a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. The table shows additional similarities and. Both cells have electrical current flowing. electrons in galvanic cells (several cells together comprise a battery). Similarities Between Galvanic And Electrolytic Cells.
From sites.google.com
week 914 chemistry Similarities Between Galvanic And Electrolytic Cells electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. Both cells have electrical current flowing. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. — the main distinction is that galvanic cells convert chemical energy into. Similarities Between Galvanic And Electrolytic Cells.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube Similarities Between Galvanic And Electrolytic Cells — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. The table shows additional similarities and. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. a galvanic cell is an electrochemical. Similarities Between Galvanic And Electrolytic Cells.
From exoyksicy.blob.core.windows.net
Distinguish Between Galvanic Cell And Electrolytic Cell at John Rowe blog Similarities Between Galvanic And Electrolytic Cells a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. Both cells have electrical current flowing. — electrolytic cells are very similar to voltaic (galvanic) cells in the. Similarities Between Galvanic And Electrolytic Cells.
From www.vrogue.co
Difference Between Galvanic Cells And Electrolytic Ce vrogue.co Similarities Between Galvanic And Electrolytic Cells a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. — the galvanic cell and electrolytic cells have some similarities. — electrolytic cells are very similar to voltaic. Similarities Between Galvanic And Electrolytic Cells.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID1196366 Similarities Between Galvanic And Electrolytic Cells — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of the. — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a. Similarities Between Galvanic And Electrolytic Cells.
From employees.csbsju.edu
CHEM 123 GENERAL CHEMISTRY 1 Similarities Between Galvanic And Electrolytic Cells The table shows additional similarities and. — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. Both cells have electrical current flowing. — the galvanic cell and electrolytic cells have some similarities. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons.. Similarities Between Galvanic And Electrolytic Cells.
From www.youtube.com
Galvanic Cell Vs Electrolytic Cell differences YouTube Similarities Between Galvanic And Electrolytic Cells a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. —. Similarities Between Galvanic And Electrolytic Cells.
From www.knowswhy.com
Similarities Between Galvanic and Electrolytic Cell Similarities Between Galvanic And Electrolytic Cells — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. — the galvanic cell and electrolytic cells have some similarities. The table shows additional similarities and. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Similarities Between Galvanic And Electrolytic Cells.
From www.chemca.in
Types of Electrochemical Cells Difference between galvanic cell and Similarities Between Galvanic And Electrolytic Cells — the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. The table shows additional similarities and. — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. Both cells have electrical current flowing. electrons in galvanic. Similarities Between Galvanic And Electrolytic Cells.
From jesse-has-lozano.blogspot.com
Explain the Difference Between a Galvanic Cell and Electrolytic Cell Similarities Between Galvanic And Electrolytic Cells Both cells have electrical current flowing. — the galvanic cell and electrolytic cells have some similarities. — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. The table shows additional similarities and. a galvanic (voltaic) cell uses the energy released during. Similarities Between Galvanic And Electrolytic Cells.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Similarities Between Galvanic And Electrolytic Cells — electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. Both cells have electrical current flowing. — the galvanic cell and electrolytic cells have some similarities. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Similarities Between Galvanic And Electrolytic Cells.
From www.youtube.com
Similarities and Differences Between Galvanic and Electrolytic Cells Similarities Between Galvanic And Electrolytic Cells a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell. electrons in galvanic cells (several cells together comprise a battery) have higher potential energy at one terminal of. Similarities Between Galvanic And Electrolytic Cells.